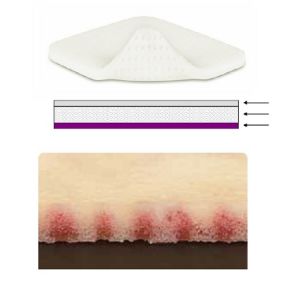
Mepilex XT ABSORBENT DRESSING IN POLYURETHANE FOAM WITH
EXCLUSIVE CHANNELS DESCRIPTION Absorbent polyurethane foam dressing with soft silicone contact layer with selective micro-adhesion.
INTENDED USE Soft silicone dressing that absorbs both low and high viscosity exudates.
Indicated for a wide range of acute and chronic injuries in all healing phases such as:
> ulcers of the lower limbs;
> pressure ulcers;
> traumatic injuries (example: skin tears);
> wounds that heal by secondary intention.
COMPOSITION The composition of the product is as follows:
1) External film in polyurethane permeable to water vapor and impermeable to liquids and bacteria.
2) Flexible and absorbent pad of open cell polyurethane foam with vertical absorption equipped with innovative channels for the collection of any type of exudate (Figure below).
3) Soft silicone layer in contact with the wound (Safetac technology).
MECHANISM OF ACTION > The action of the product determines a control of both low and high viscosity exudates and the maintenance of the ideal moist environment for wound healing.
> Absorbs exudates vertically through innovative channels that allow the collection of any type of exudate.
> Thanks to the Safetac technology contact layer, it minimizes removal trauma, protects the periwound skin, thus preventing maceration.
PRODUCT BENEFITS > It can be removed and repositioned without losing its selective micro-adhesion characteristics.
> It can be left in place for several days (up to seven); the time period may vary based on the condition of the wound and periwound skin or as indicated by routine clinical procedures.
> Flexible, conformable and can be cut.
Safetac Technology Patented technology in soft silicone, applied to Mölnlycke Health Care dressings, which minimizes pain to patients and trauma to the injury even in critical situations, when the exudate begins to dry:
> does not adhere to moist surfaces such as open wounds but to dry wound edges;
> adapts to the uneven surface of the skin to create a greater contact area, which allows for better distribution of the force necessary for removal, preventing skin stripping;
> seals the edges of the lesion, preventing the exudate from spreading on the periwound skin, minimizing the risk of maceration;
> leaves no residue either on the wound bed or on the periwound skin. METHOD OF USE > Clean the wound according to your usual procedures and allow the periwound skin to dry.
> Open the package and remove the protective paper and apply the “Safetac” side of the dressing to the wound. Do not stretch the dressing.
> For best results Mepilex XT must cover the periwound skin for at least 1-2 cm beyond the edges of the wound for the small sizes (sizes up to 10 x 10 cm) and for at least 5 cm for the larger sizes, in order to protect the periwound skin from maceration and excoriation and to be able to fix the dressing safely. If necessary, the product can be cut to size to fit different shapes and anatomical areas.
> If necessary, secure with a bandage or other fastening system.
> Can be used under compression bandages and in combination with gels.
> Initially it may be necessary to change the dressing more frequently as a change in treatment may lead to an initial increase in exudate.
> Maintains the ideal moist environment for healing and promotes wound debridement. Initial enlargement of the wound size may occur. This effect is completely normal.
CONTRAINDICATIONS / PRECAUTIONS FOR USE > For external use.
> In case of sensitization of the treated area or clinical signs of infection, consult the healthcare professional.
> Do not use on patients with known sensitivity to the dressing or its components.
> Do not use in conjunction with oxidizing agents such as hypochlorite solutions or hydrogen peroxide.
> The polyurethane foam used in the product can change color to yellow when exposed to light, air and / or heat. The color variation does not affect the properties of the product, if used within the expiration date.
TECHNICAL FEATURES Thickness: 5.6 mm
Weight: 859 g / m
2 | Test | Result | Applied method |
| Permeability to water vapor: (MVTR) at 24h | 23 g / 10 cm 2/24 h
equal to 23000 g / m 2/24 h | EN 13726 -1 3.3 |
| Absorption: | 7 g / 10cm 2/24 h
equal to 7000 g / m 2/24 h | EN 13726 - 1 3.3 |
| Fluid management skills | 32 g / 10cm 2/24 h
equal to 32,000 / m 2/24 h | EN 13726 - 1 |
| Retention capacity under compression (40mmHg) | 5.6g / g
0.40g / cm ² | Internal method
MHC |
Resistance to penetration * by viruses and bacteria
* micro organisms larger than 25 nm | Approved | EN 13795-4,
ASTM F1671 |
BIOCOMPATIBILITY Mepilex XT has successfully passed the following biocompatibility tests.
| Test | Result | Standard applied |
| Cytotoxicity | Not cytotoxic | EN 10993 |
| Allergic sensitization | Not sensitizing | EN 10993 |
| Skin irritation | Not irritating | EN 10993 |
| Systemic toxicity | Non toxic | EN 10993 |
| Genotoxicity | Not genotoxic | EN 10993 |
STERILITY and STORAGE > Sterilized with ethylene oxide with a process validated according to EN 550: 1994, ISO 11135: 1994 and ISO 109937: 1995 for EtO residues.
> The sterility of the individual packaging is guaranteed for 3 years, provided that it is intact, i.e. it has not been opened or damaged.
> Do not resterilize.
Do not use after the expiration date. Product properties are not guaranteed if used after the expiration date.
Not reusable. Reuse of the product may compromise its effectiveness and cause cross-contamination.
LABELING It is a product compliant with EEC directive 93/42
MANUFACTURER Made in Finland by Mölnlycke Health Care AB, Gothenburg (Sweden)
ENVIRONMENTAL AND QUALITY CERTIFICATIONS CE marking - EEC Directive 93/42 and subsequent amendments.
Quality and environmental standards applied:
>
EN ISO 9001: 2000 and
ISO 13485: 2003 .
>
EN ISO 14001: 2004 referring to environmental management systems
>
ISO 14791: 2007 referred to product Risk Management
STORAGE, VALIDITY AND DISPOSAL > The validity period is 3 years. Do not use after the expiration date. Product properties are not guaranteed if used after the expiration date.
> Store at a temperature below 35 ° C (95 ° F), in a dry place and away from direct sunlight.
> Its disposal must be carried out in accordance with local environmental protection procedures.
ASSORTMENT AND PACKAGING Packaging on 3 levels:
>
Transport Packaging: made of corrugated and reinforced cardboard, it ensures protection from external agents and the integrity of the product during transport.
>
Dispenser: made of lighter and more flexible cardboard, its purpose is to protect the product on the shelves of the department until it is used.
>
Single sterile protective pouch: made up of a transparent film and paper, it ensures the sterility of the product (if intact) until the dressing is used on the patient.
>
The product and packaging are Latex Free Mepilex XT is sterile packed in a single pouch
CND M04040602 |
| Code | Dimension
total (cm) | Pieces
/ dispenser | Pieces / pack | Repertoire Code |
| 211015 | 5 X 5 | 5 | 40 | 1333037 / R |
| 211100 | 10 x 10 | 5 | 70 | 1242368 / R |
| 211200 | 10 x 20 | 5 | 45 | 1242369 / R |
| 211300 | 15 x 15 | 5 | 25 | 1242370 / R |
| 211400 | 20 x 20 | 5 | 20 | 1242371 / R |
Cod. 211100